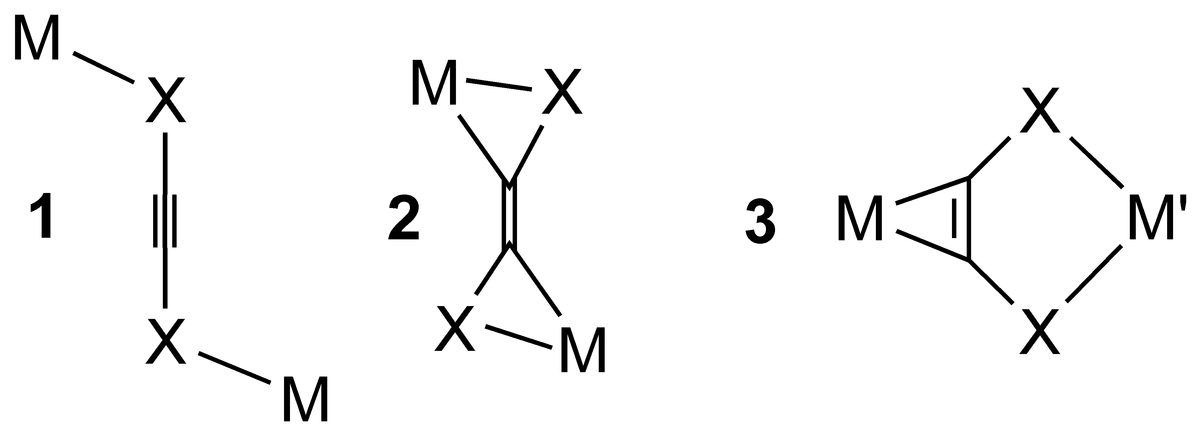Donor-substituted Alkynes in Polynuclear Complexes
We are dedicated to the development of a coordination chemistry with α-donor substituted alkynes [X-C≡C-X]n- (X = O, S, NR, PR2, n = 0, 1, 2). These ligands can bridge two metals in different coordination modes including linear μ-k2-X,X’ (1), μ-η2-C,X-η2-C’,X’- (2) as well as the μ-η2-C,C'-k2-X,X' arrangement (3). Bonding mode 1 in particular is suitable for linking different metals, since the organometallic side-on coordination meets significantly different demands of a metal than the Werner-type chelate coordination. In addition, variation of the donor set allows adaption to the requirements of the second metal. Ultimately short metal-to-metal distances combined with the unsaturated nature of the bridging system support a strong electronic cooperativity of the linked metals.
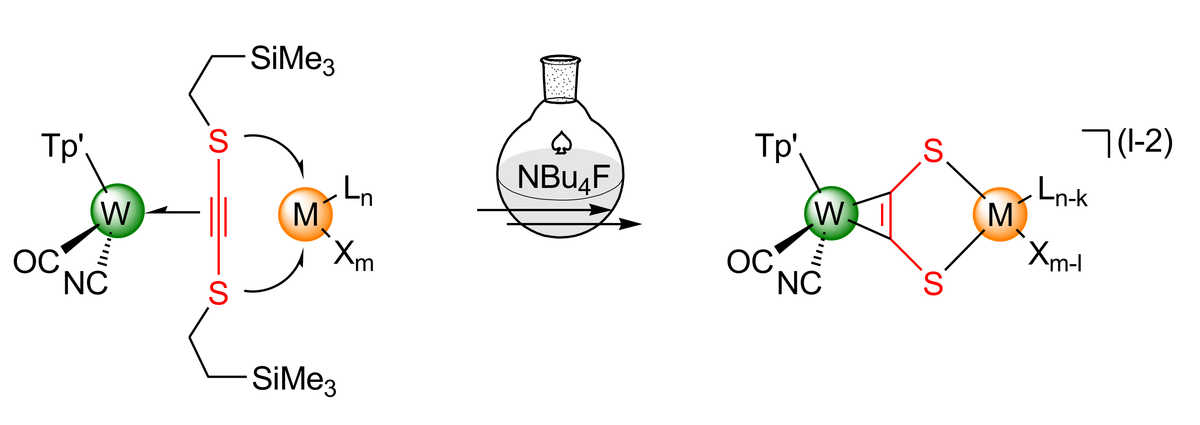
A basic focus of our work is the exploration of synthetic concepts. Many donor alkynes must be generated in the primarily formed alkyne complex, since most of these electron-rich alkyne derivatives are intrinsically unstable. Furthermore, we study the electronic structure of the complexes obtained by means of spectroscopic and theoretical methods (NMR, IR, rRaman, UV-vis/NIR, EPR, cyclic voltammetry, DFT). Depending on the donor type either delocalized mixed-valent complexes or localized systems displaying redox isomerism are observed. In addition, the general redox activity of early-transition-metal alkyne complexes makes corresponding metalla-η2-C,C'-donor alkyne complexes promising as functional κ2-X,X' ligands for high electron-demand or redox-switchable catalysis (e.g. for X=PR2). The variability of the donor atoms should allow here a wide range of applications.
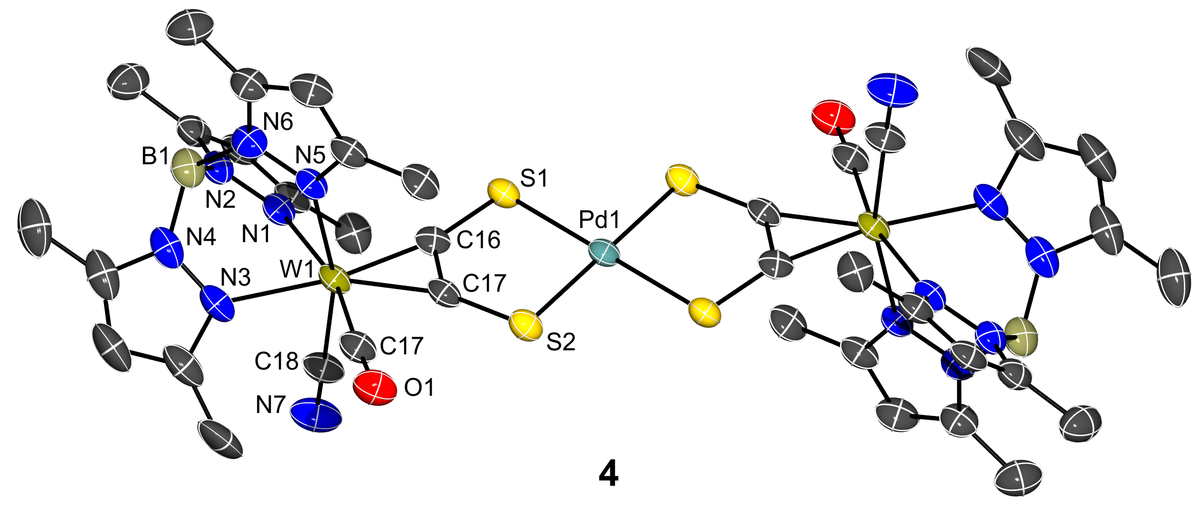
The Figures show a dianionic trinuclear PdW2 complex 4 displaying a square planar coordinated Pd center and a nano-scale Cu4W4 supramolecular complex, in which four W-alkyne complex moieties and a Cu4 tetrahedron are held together by four acetylene-dithiolate ligands (acdt2-).
Key Publications
- "Synthesis and Activation Potential of an Open Shell Diphosphine"
K. Helmdach, S. Ludwig, A. Villinger, D. Hollmann, J. Kösters, W. W. Seidel
Chem. Commun. 2017, 53, 5894–5897. - "Minimalistic Ditopic Ligands: An α-S,N-Donor-Substituted Alkyne as Effective Intermetallic Conjugation Linker"
J. Rüger, C. Timmermann, A. Villinger, A. Hinz, D. Hollmann, W. W. Seidel
Chem. - Eur. J.2016, 22, 11191–11195. - "Dikohlenstoffdisulfid (S=C=C=S): Stabilisierung und Isomerisierung in einem Cobaltkomplex"
W. W. Seidel, M. J. Meel, S. R. Hughes, F. Hupka, A. Villinger
Angew. Chem.2011, 123, 12825–12828;
Angew. Chem. Int. Ed.2011, 50, 12617–12620. - "On the Prospects of Polynuclear Complexes with Acetylenedithiolate Bridging Units"
W. W. Seidel, M. J. Meel, U. Radius, M. Schaffrath, T. Pape
Inorg. Chem.2007, 46, 9616–9629. - "Kann Acetylendithiolat als vierzähniger Brückenligand wirken?"
W. W. Seidel, M. Schaffrath, T. Pape
Angew. Chem. 2005, 117, 7976–7979;
Angew. Chem. Int. Ed. 2005, 44, 7798–7800.