Analytical methods at the Seidel group
Due to the research focus of the Seidel group between organometallics and coordination chemistry, numerous analytical methods are available to examine the synthesised systems. Based on these results, conclusions can be drawn about the electronic situation, the ligand environment in the complex or the composition of the novel compounds, among other things.
The following is an excerpt of analytical methods with the corresponding equipment that are available and carried out in the Seidel group. Other necessary analyses are usually available in-house or are carried out with the help of cooperation partners.
If you have any questions about the analytical methods or the equipment, please contact the person in charge or Prof. Dr. Wolfram W. Seidel.
Mass spectrometry
Mass spectrometric measurements are ubiquitous in chemistry! Important information about the composition of samples can be obtained in all areas. Especially in preparative molecular chemistry, it is necessary to use mass spectrometric methods to unambiguously characterise chemical compounds and to elucidate their structure.
In preparative inorganic chemistry, chemical compounds with very different properties are synthesised, which means that the corresponding characterisation methods must have a wide range of applications. Since October 2020, the inorganic chemistry department at the University of Rostock has had a comprehensive mass spectrometer for the investigation and characterisation of different chemical compounds.
The different ionisation methods using electron spray ionisation (ESI) and chemical ionisation at atmospheric pressure (APCI) mean that not only charged or polar complexes but also uncharged and non-polar organometallic compounds can be investigated in a mass range from 10 to 2000 m/z. The sample form plays only a subordinate role in the measurements, so reaction solutions can be injected directly into the ionisation source, among other things. This method is particularly suitable for monitoring the course of the reaction when ligand and metal precursor are coordinated in order to draw conclusions about the conversion and formation of the product. In addition, solids can also be ionised directly, so that even poorly soluble organometallic compounds can be investigated.
- Instrument: expression L Compact Mass Spectrometer from Advion
- Ion source: ESI, ASAP, APCI
- Analyser: Single-Quadrupol Analyzer
- Mass range: 10 to 2000 m/z
- Supervising person: M.Sc. Lene Zabojnik
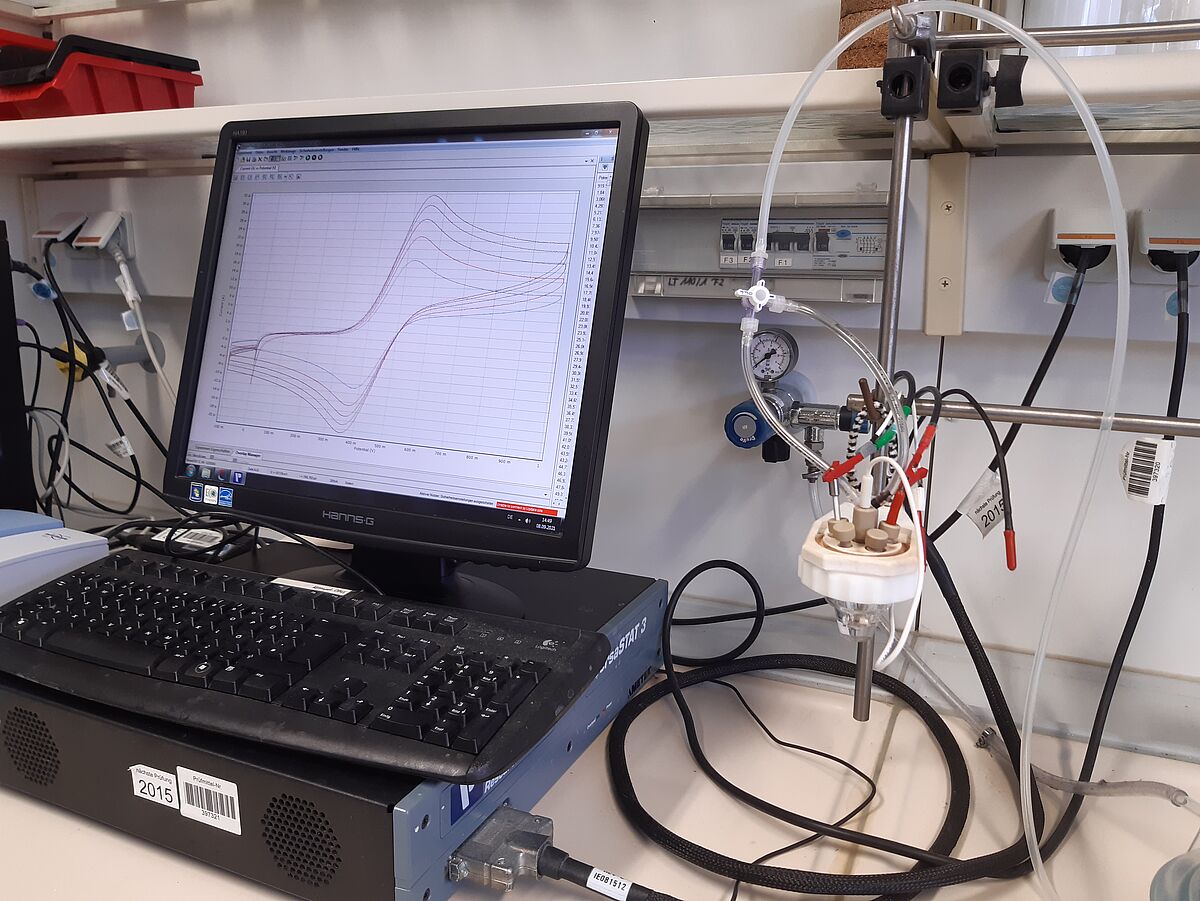
Cyclic voltammetry
Cyclic voltammetry also allows us to analyse the electrochemical properties of our complex compounds. The redox processes around the various metal centres are often of particular interest. We use the Versa-STAT3 as a potentiostat and can choose from various working electrodes (Pt, Au, C). As we can also measure under argon as an inert gas, a wide variety of compounds can be characterised.
- Instrument: VersaStat3 from Ametek
- Working electrode: platinum, gold oder carbon
- reference electrode: Ag/Ag+
- Counter electrode: platinum
- Supervising person: M.Sc. Friederike Hamann
Infrared spectroscopy
Infrared spectroscopy is one of the essential analytical methods in our working group and has a wide range of applications. With the ALPHA T IR spectroscope from Bruker, it is possible to carry out measurements directly in the laboratory. For example, reaction monitoring using IR spectroscopy is a method of gaining direct insights into the chemical reaction. A wide range of solvents can be covered with different IR cells, allowing numerous solutions and compounds to be identified and characterised.
- Instrument: ALPHA T from Bruker
- Infrared range: 400 cm-1 to 4000 cm-1
- Cell window: KBr
- Supervising person: M.Sc Malte Reihwald
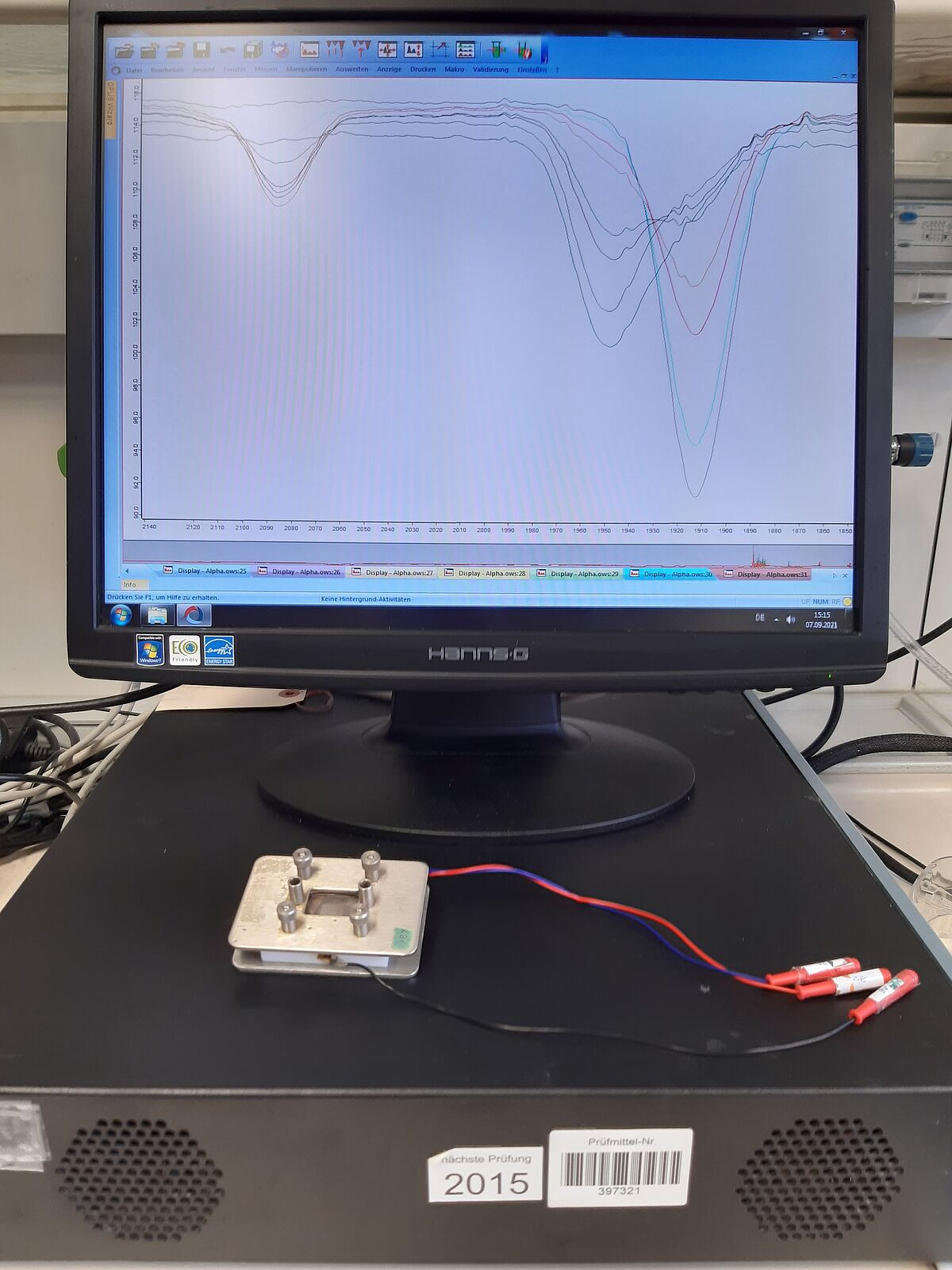
Spectroelectrochemistry (SEC)
We combine the methods of infrared spectroscopy and cyclic voltammetry in spectroelectrochemical measurements. This means that electrochemical redox processes can also be monitored spectroscopically at the same time. This allows further insights into the electronic properties of individual complexes, as well as into mechanisms and subsequent reactions. The ALPHA T IR spectroscope from Bruker and the Versa-STAT3 potentiostat are also used for measurements, as well as a self-built measuring cell equipped with Pt electrodes.
- Instrument: ALPHA T from Bruker and VersaStat3 from Ametek
- Working electrode: platinium
- Counter electrode: platinium
- Reference electrode: Ag/Ag+
- Cell window: KBr
- Revising person: M.Sc. Friederike Hamann
Raman- and Resonance-Raman-spectroscopy
Raman spectroscopy is a good addition to infrared spectroscopy for analysing vibrational modes. While vibrations are observed in infrared spectroscopy due to changes in the dipole moment, changes in polarisability are necessary to detect vibrations in Raman spectroscopy.
- Instrument: LabRAM HR from Jobin Yvon Technologies
- Available LASER: 473 nm, 532 nm, 633 nm and 785 nm
- Supervising person: M.Sc. Lene Zabojnik
UV/Vis-spectroscopy
UV/Vis spectroscopy has always been one of the most important methods of spectroscopy in chemistry. This quantitative analysis method is not only used to determine concentrations of solutions, but also to obtain information about electronic situations within a chemical compound.
UV/Vis spectroscopy plays a particularly important role in organometallic chemistry. It allows to make statements about the colour of a complex as well as about charge transfer processes between metal centres and ligands.
- Instrument: Cary 60 UV-Vis from Agilent Technologies
- Wavelength range: 200 nm to 1200 nm
- Supervising person: M.Sc. Friederike Hamann and M.Sc. Malte Reihwald
Fluorescence spectroscopy
Another important analytical method is fluorescence spectroscopy. In this spectroscopy method, fluorescence phenomena in chemical compounds are utilised to carry out qualitative and quantitative investigations. In addition to applications in organic chemistry, biochemistry and medicine, fluorescence spectroscopic analyses are also used in organometallic chemistry. For example, Ru(II) complexes of the type [(bipy)2Ru(phen)][PF6]2 show clear fluorescence. This allows statements to be made about the excited state, the quantum yield and the use as a photocatalyst.[1]
[1] W. W. Seidel et al., Photochem. Photobiol. Sci. 2018, 17, 1056.
- Instrument: Cary Egilent Fluorescence Spectrophotometer from Agilent Technologies
- Wavelength range: 200 nm to 1200 nm
- Supervising person: Prof. Dr. Wolfram W. Seidel