Metal Controlled C-C Coupling Reactions
In many cases, alkynes at late transition metals are prone to C/C coupling reactions. Hetero-substitution of such alkynes in comparison to carbon-based ones has not only a substantial influence on the rates but also on the outcome of the couplings. Hence, unusual coupling products like folded rhenacyclopentatrienes could be obtained.
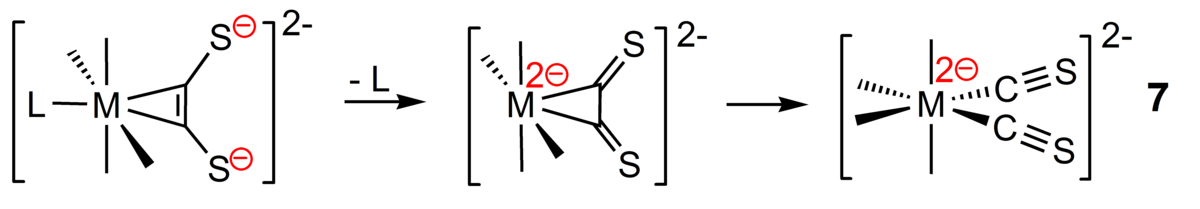
A long-term and more fundamental project involves the generation of hitherto unknown homoleptic thiocarbonyl complexes starting with alkyne complexes of acetylenedithiolate. Previous attempts in this direction using in situ generated CS turned out to be cumbersome. Under certain conditions, such as labile co-ligands and suitable oxidation states, however, alkyne complexes of acetylenedithiolate should be converted into bis(thiocarbonyl) complexes. Comparable reactivity has thoroughly been investigated for related ligands such as isocyanides and CO. Utilization of homoleptic bis (alkyne) complexes (Ni, Pt) or corresponding tris(alkyne)complexes (Mo, W) could potentially open access to novel poly(thiocarbonyl) complexes.
Key Publications
- "Spontaneous Formation of an η4-Ethylene Bis(carbene) Ligand by Alkyne Coupling at Rhenium(III)"
S. Kleinschmidt, D. Schallenberg, K. Helmdach, A. Hinz, A. Villinger, W. W. Seidel
Organometallics2015, 34, 1091–1097. - "Facile Formation of a Rhenium Allenylcarbene Complex with an Internal Dithioalkyne"
W. W. Seidel, M. J. Meel, D. Schallenberg, T. Pape, A. Villinger, D. Michalik
Eur. J. Inorg. Chem.2010, 5523–5528.